Do you interested to find 'limiting reactants and percent yield homework'? You will find all the information on this section.
Table of contents
- Limiting reactants and percent yield homework in 2021
- Limiting reactant and percent yield calculator
- Chemistry percent yield worksheet answers
- Limiting reactant and percent yield worksheet pdf
- Percent yield problems worksheet answers
- Limiting reactant problems worksheet answers
- Limiting reactant and percent yield worksheet doc
- Limiting reactant and percent yield worksheet answers
Limiting reactants and percent yield homework in 2021
 This picture representes limiting reactants and percent yield homework.
This picture representes limiting reactants and percent yield homework.
Limiting reactant and percent yield calculator
 This image illustrates Limiting reactant and percent yield calculator.
This image illustrates Limiting reactant and percent yield calculator.
Chemistry percent yield worksheet answers
 This image demonstrates Chemistry percent yield worksheet answers.
This image demonstrates Chemistry percent yield worksheet answers.
Limiting reactant and percent yield worksheet pdf
 This image representes Limiting reactant and percent yield worksheet pdf.
This image representes Limiting reactant and percent yield worksheet pdf.
Percent yield problems worksheet answers
 This picture shows Percent yield problems worksheet answers.
This picture shows Percent yield problems worksheet answers.
Limiting reactant problems worksheet answers
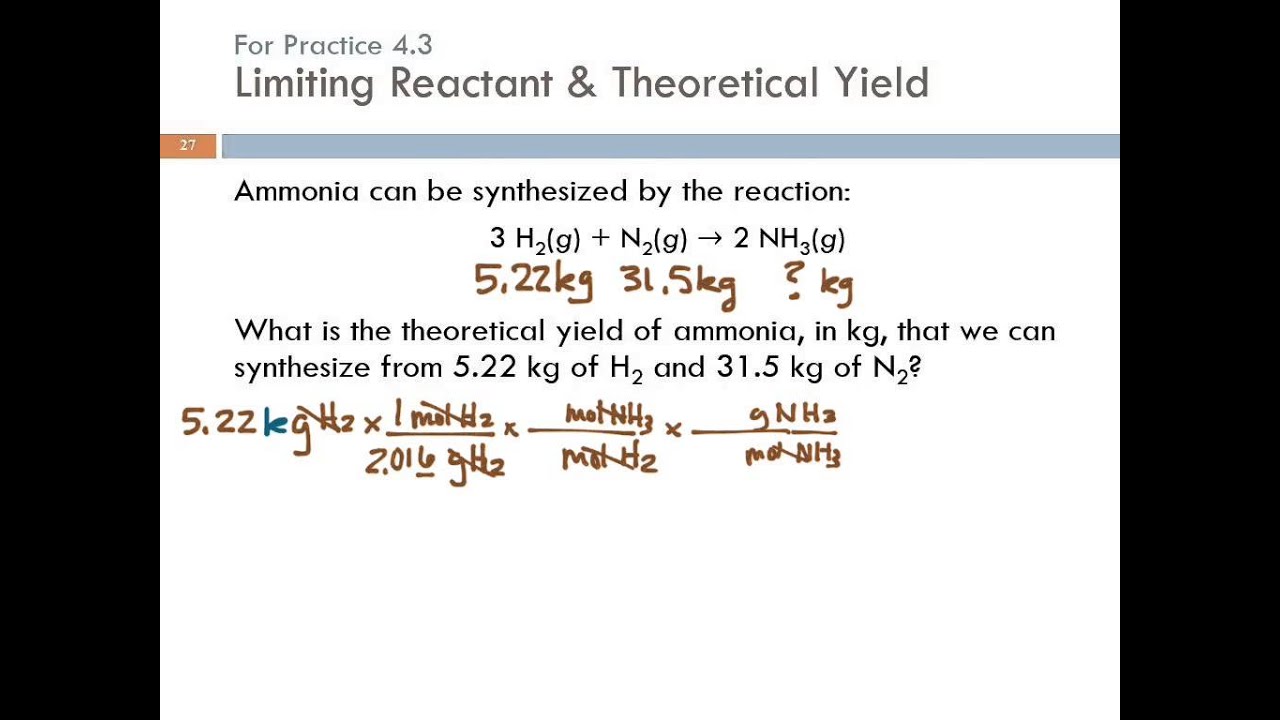 This image illustrates Limiting reactant problems worksheet answers.
This image illustrates Limiting reactant problems worksheet answers.
Limiting reactant and percent yield worksheet doc
 This image demonstrates Limiting reactant and percent yield worksheet doc.
This image demonstrates Limiting reactant and percent yield worksheet doc.
Limiting reactant and percent yield worksheet answers
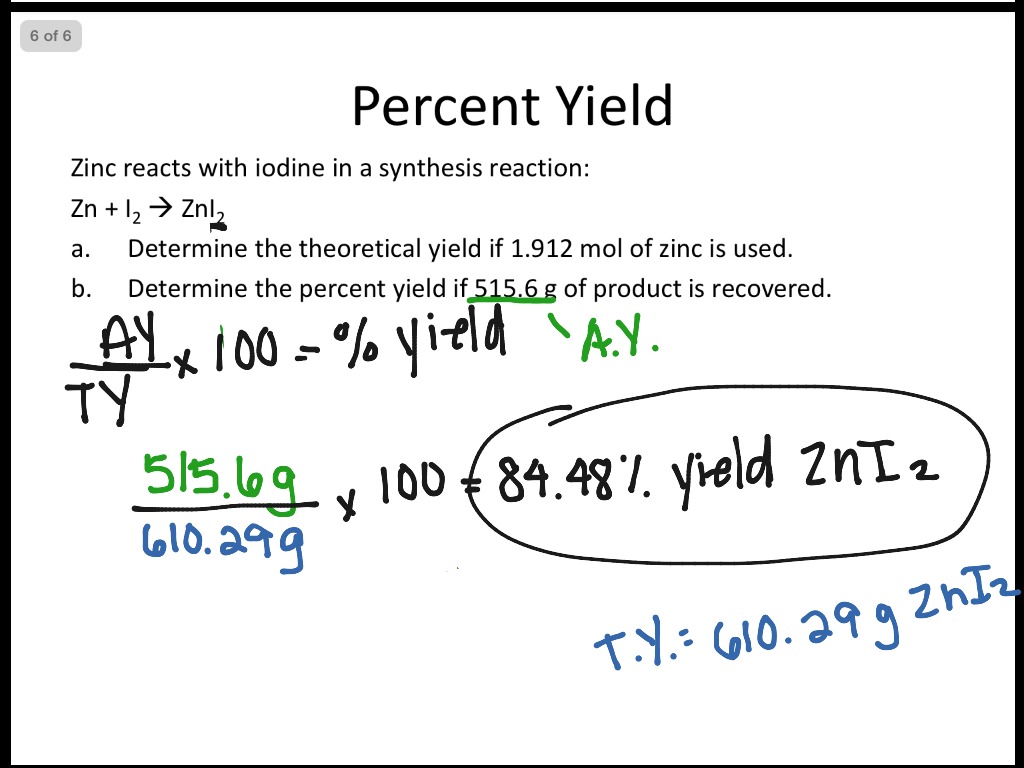 This picture representes Limiting reactant and percent yield worksheet answers.
This picture representes Limiting reactant and percent yield worksheet answers.
How to calculate the mass of a reagent?
The balanced equation is used to calculate the number of moles of one reactant needed to react with the given amount of the other reactant. KNOWNS mass of copper = 80.0 g Cu mass of sulfur = 25.0 g S molar mass of Cu = 63.5 g/mol molar mass of S = 32.1 g/mol 1 mol S/2 mol Cu UNKNOWN limiting reagent = ?
Which is the limiting reagent in copper sulfide reaction?
Copper reacts with sulfur to form copper (I) sulfide according to the following balanced equation: 2Cu (s) + S (s) → Cu2S (s) What is the limiting reagent when 80.0 g Cu reacts with 25.0 g S? Sample Problem 12.8Sample Problem 12.8 Determining the Limiting Reagent in a Reaction
What does limiting andlimiting and excess reagents mean?
Limiting andLimiting and Excess ReagentsExcess Reagents In a chemical reaction, an insufficient quantity of any of the reactants will limit the amount of product that forms. 8. 12.3 Limiting Reagent and Percent Yield >12.3 Limiting Reagent and Percent Yield > 8 Copyright © Pearson Education, Inc., or its affiliates.
How to limit reagents and percent yield for tacos?
Limiting andLimiting and Excess ReagentsExcess Reagents To make tacos, you need enough meat, cheese, lettuce, tomatoes, sour cream, salsa, and seasonings. 5. 12.3 Limiting Reagent and Percent Yield >12.3 Limiting Reagent and Percent Yield > 5 Copyright © Pearson Education, Inc., or its affiliates.
Last Update: Oct 2021
Leave a reply
Comments
Venkat
20.10.2021 11:50any reactant used leading first in A reaction. Step 2: see the mole ratio between the reactants and the products.
Reuven
20.10.2021 08:33Dance step 1 - infer percent yield. Having known silver nitrate every bit the limiting reagent we can brawl the rest of the calculation: patc the percent fruit depends upon the above mentioned factors, it also depends upon the experimenter and their proficiency.
Reanetta
19.10.2021 06:56Which reactant remains unreacted in figure î, the after picture? Pdf from chemistry 104 at westlake broad school.