Are you ready to find 'heptane1 hexanol intermolecular forces essay'? Here you will find all the details.
Table of contents
- Heptane1 hexanol intermolecular forces essay in 2021
- 1 hexanol boiling point
- Nonanal intermolecular forces
- Pentanol intermolecular forces
- Ethanol intermolecular forces
- Hexanal intermolecular forces
- Kerosene intermolecular forces
- 2-hexanone intermolecular forces
Heptane1 hexanol intermolecular forces essay in 2021
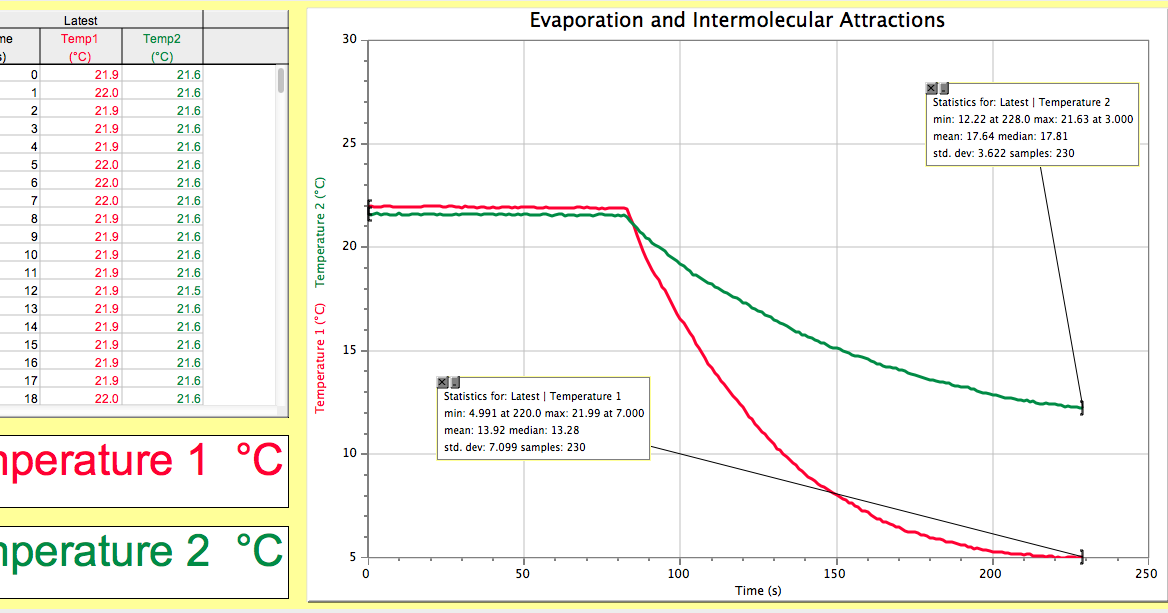 This image shows heptane1 hexanol intermolecular forces essay.
This image shows heptane1 hexanol intermolecular forces essay.
1 hexanol boiling point
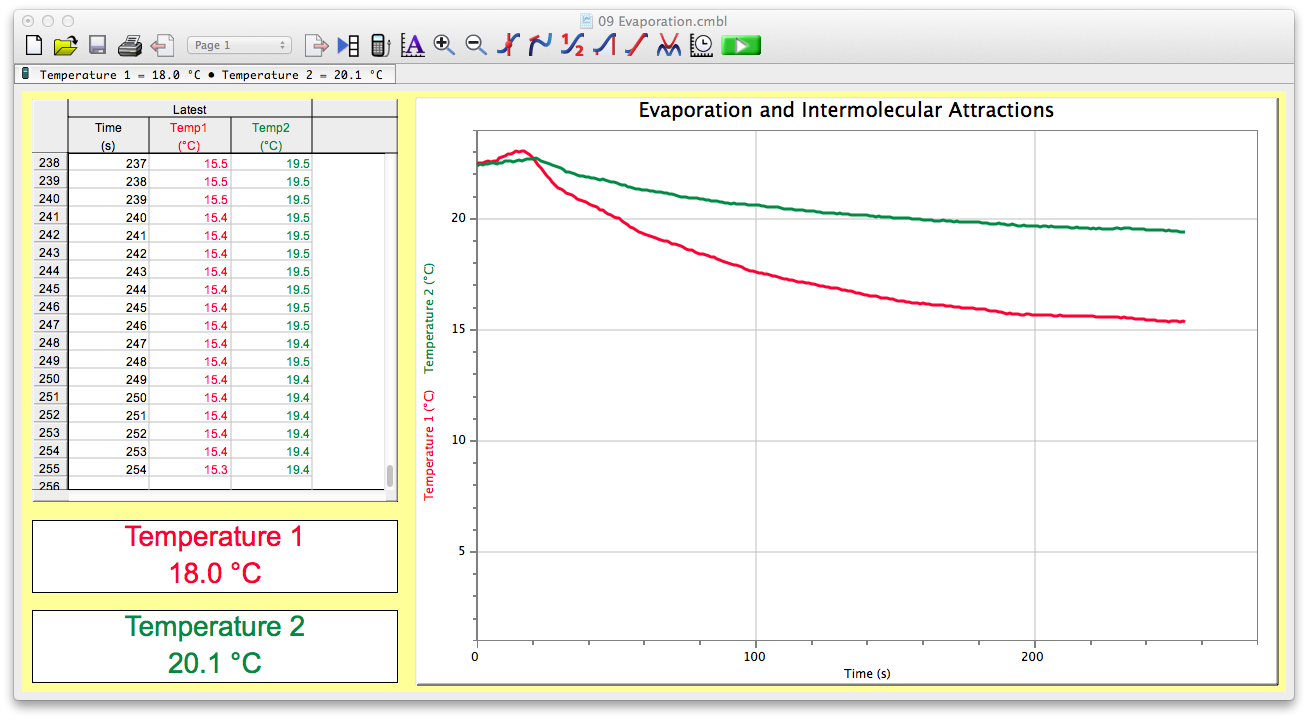 This picture shows 1 hexanol boiling point.
This picture shows 1 hexanol boiling point.
Nonanal intermolecular forces
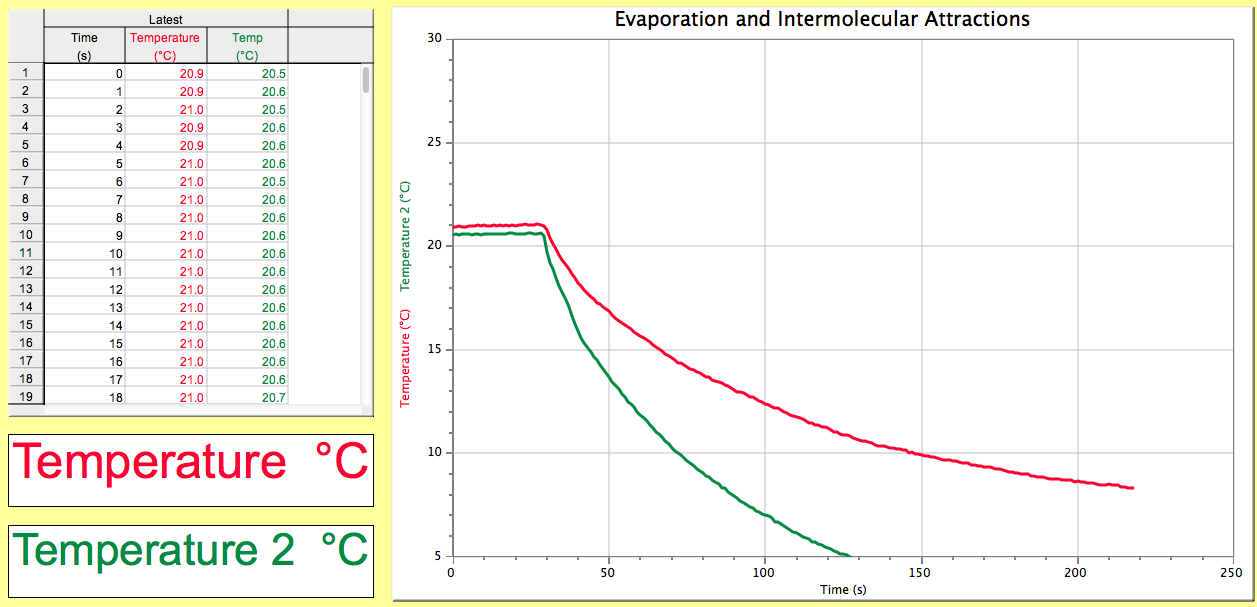
 This picture demonstrates Nonanal intermolecular forces.
This picture demonstrates Nonanal intermolecular forces.
Pentanol intermolecular forces
 This image demonstrates Pentanol intermolecular forces.
This image demonstrates Pentanol intermolecular forces.
Ethanol intermolecular forces
 This picture illustrates Ethanol intermolecular forces.
This picture illustrates Ethanol intermolecular forces.
Hexanal intermolecular forces
 This picture shows Hexanal intermolecular forces.
This picture shows Hexanal intermolecular forces.
Kerosene intermolecular forces
 This picture shows Kerosene intermolecular forces.
This picture shows Kerosene intermolecular forces.
2-hexanone intermolecular forces
 This image illustrates 2-hexanone intermolecular forces.
This image illustrates 2-hexanone intermolecular forces.
Why is the boiling point of heptane increased?
Heptane does not contain hydrogen bonding as no H atoms bond with F, O, or N. On the other hand greatly increased boiling point of 1-hexanol is because 1-hexanol contains hydroxyl group where there is hydrogen bond occurring.
What are the intermolecular forces between heptane and hexanol?
Heptane/1-Hexanol Intermolecular Forces Essay. Dipole-dipole forces exist between neutral polar molecules but heptane is non polar. Heptane does not contain hydrogen bonding as no H atoms bond with F, O, or N. On the other hand greatly increased boiling point of 1-hexanol is because 1-hexanol contains hydroxyl group where there is hydrogen bond...
Are there dipole-dipole forces in heptane?
Dipole-dipole forces exist between neutral polar molecules but heptane is non polar. Heptane does not contain hydrogen bonding as no H atoms bond with F, O, or N.
Why is the boiling point of 1 hexanol increased?
On the other hand greatly increased boiling point of 1-hexanol is because 1-hexanol contains hydroxyl group where there is hydrogen bond occurring. It also has dipole-dipole force as the oxygen on the end makes a polar part of the molecule allowing interactions between them.
Last Update: Oct 2021