Do you interested to find 'double replacement metathesis reactions answers'? Here you can find your answers.
What is a bivalent replacement reaction? Bivalent replacement reactions —also called double deracination, exchange, or double decomposition reaction reactions —occur when parts of cardinal ionic compounds ar exchanged, making cardinal new compounds. The overall pattern of a double renewal reaction looks alike this:
Table of contents
- Double replacement metathesis reactions answers in 2021
- Zinc acetate + cesium hydroxide
- Metathesis reaction lab
- Metathesis reaction calculator
- Aqueous solutions of strontium hydroxide and ammonium sulfide are mixed
- Salt metathesis reaction
- Ammonium sulfide + hydrochloric acid net ionic equation
- Ammonium sulfate and potassium hydroxide are mixed together
Double replacement metathesis reactions answers in 2021
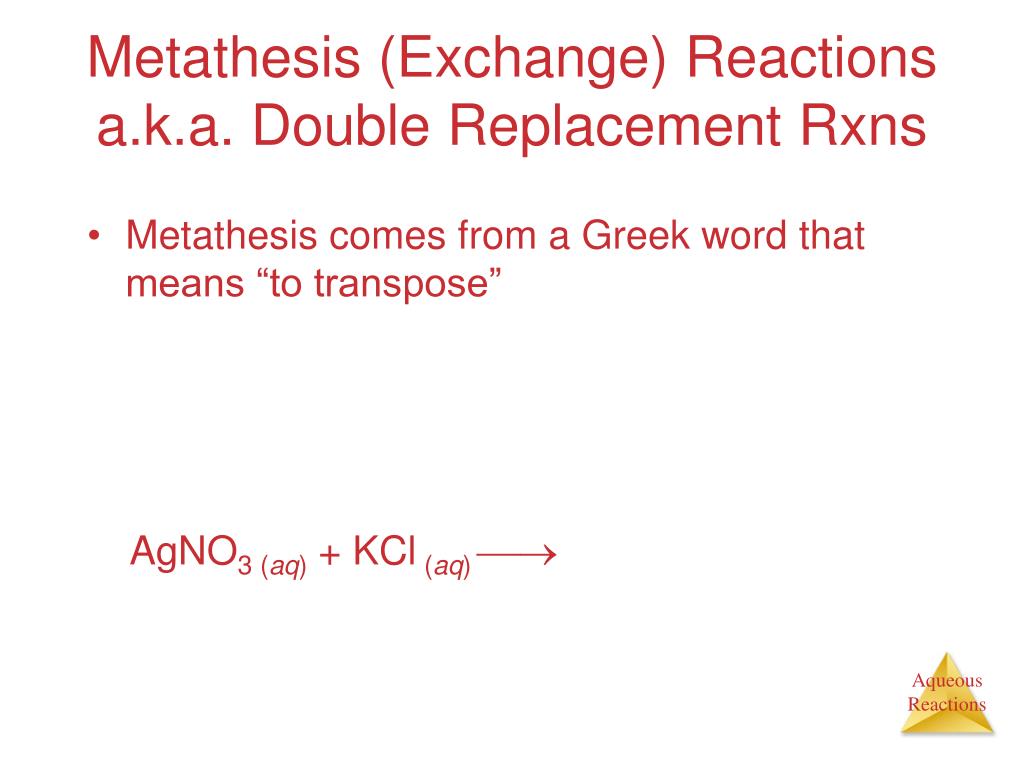 This image illustrates double replacement metathesis reactions answers.
This image illustrates double replacement metathesis reactions answers.
Zinc acetate + cesium hydroxide
 This image illustrates Zinc acetate + cesium hydroxide.
This image illustrates Zinc acetate + cesium hydroxide.
Metathesis reaction lab
 This image shows Metathesis reaction lab.
This image shows Metathesis reaction lab.
Metathesis reaction calculator
 This picture demonstrates Metathesis reaction calculator.
This picture demonstrates Metathesis reaction calculator.
Aqueous solutions of strontium hydroxide and ammonium sulfide are mixed
 This image demonstrates Aqueous solutions of strontium hydroxide and ammonium sulfide are mixed.
This image demonstrates Aqueous solutions of strontium hydroxide and ammonium sulfide are mixed.
Salt metathesis reaction
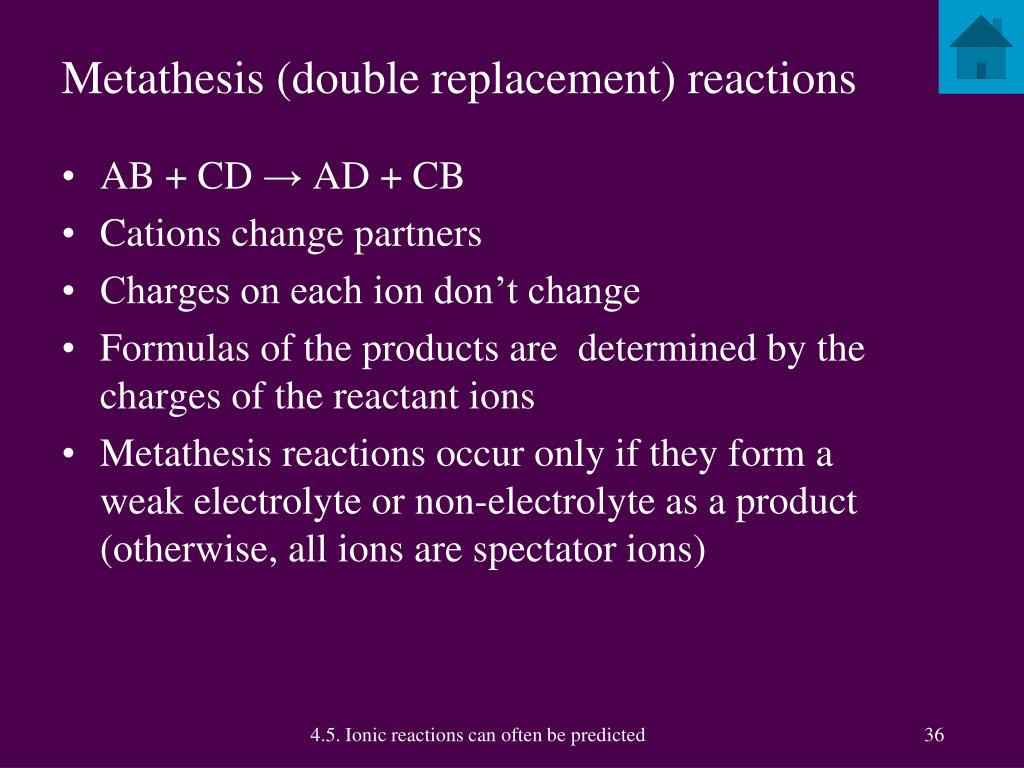 This image shows Salt metathesis reaction.
This image shows Salt metathesis reaction.
Ammonium sulfide + hydrochloric acid net ionic equation
 This image demonstrates Ammonium sulfide + hydrochloric acid net ionic equation.
This image demonstrates Ammonium sulfide + hydrochloric acid net ionic equation.
Ammonium sulfate and potassium hydroxide are mixed together
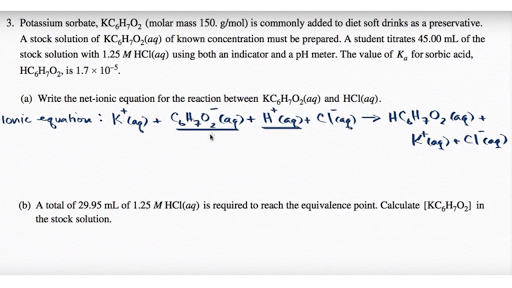 This image shows Ammonium sulfate and potassium hydroxide are mixed together.
This image shows Ammonium sulfate and potassium hydroxide are mixed together.
Which is the correct form of double displacement precipitation?
A double displacement precipitation is a reaction in which two soluble ionic compounds react to form an insoluble precipitate. Double replacement reactions take the general form: A⁺B⁻ + C⁺D⁻ → A⁺D⁻ + C⁺B⁻.
Which is an example of a metathesis reaction?
Another example is the reaction between sodium sulfide and hydrochloric acid to form sodium chloride and hydrogen sulfide: There are three classes of metathesis reactions: neutralization, precipitation, and gas formation reactions.
How can I tell if I have a double displacement reaction?
The easiest way to identify a double displacement reaction is to check to see whether or not the cations exchanged anions with each other. Another clue, if the states of matter are cited, is to look for aqueous reactants and the formation of one solid product (since the reaction typically generates a precipitate).
What is the definition of a double replacement reaction?
A double replacement reaction is a type of chemical reaction that occurs when two reactants exchange cations or anions to yield two new products. Double replacement reactions are also called double replacement reactions, double displacement reactions, or metathesis reactions.
Last Update: Oct 2021