Are you searching for 'define metathesis reaction'? You will find the answers here.
Table of contents
- Define metathesis reaction in 2021
- Metathesis chemistry definition
- Metathesis reaction vs double displacement
- Metathesis reactions lab
- Metathesis example
- Metathesis meaning in chemistry
- Metathesis reaction equation
- Metathesis green chemistry
Define metathesis reaction in 2021
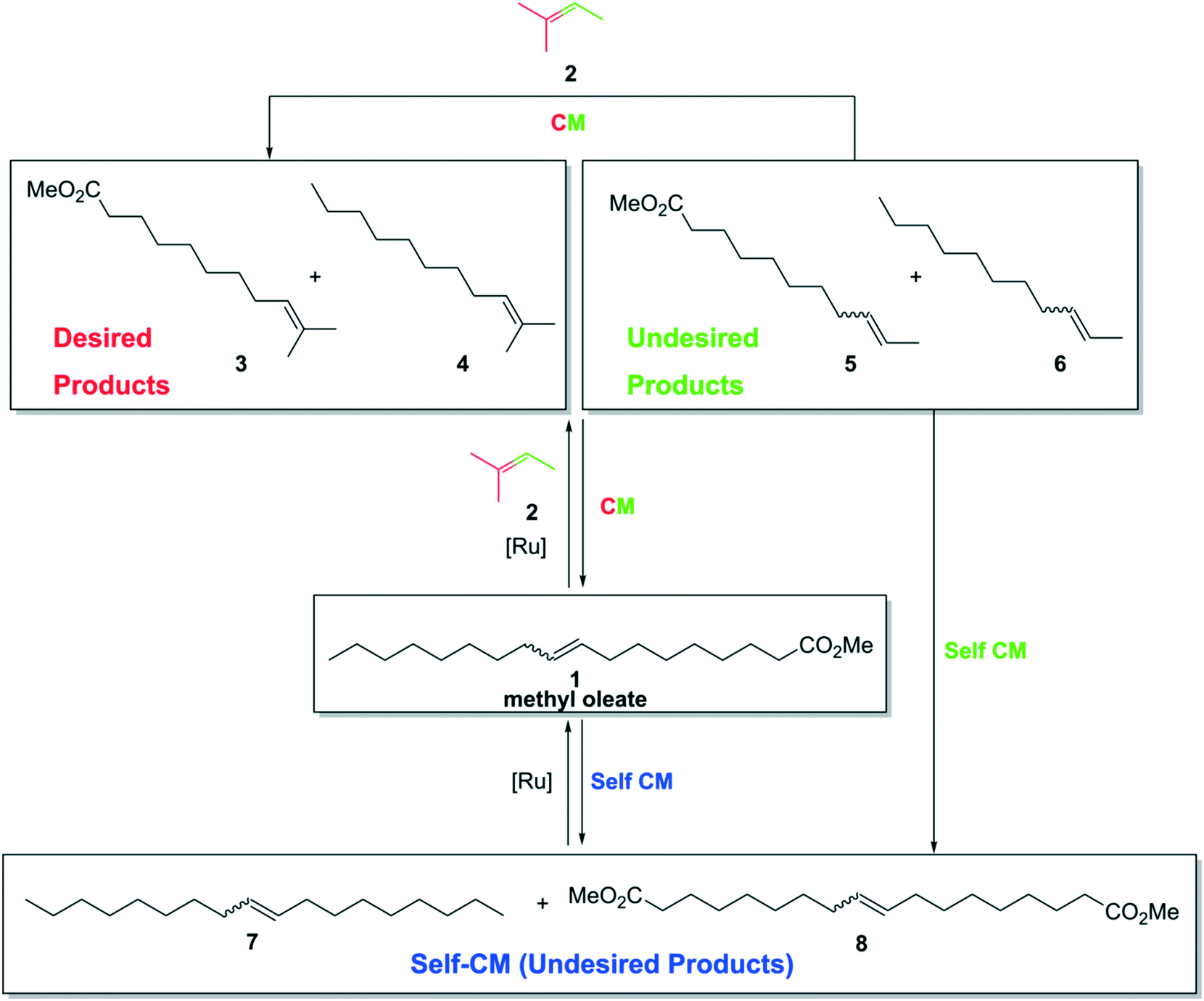 This picture illustrates define metathesis reaction.
This picture illustrates define metathesis reaction.
Metathesis chemistry definition
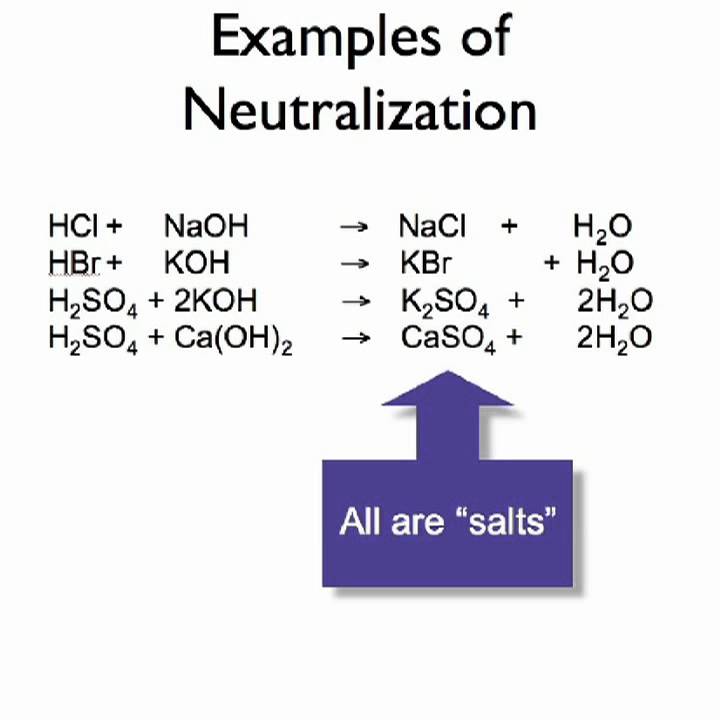 This picture representes Metathesis chemistry definition.
This picture representes Metathesis chemistry definition.
Metathesis reaction vs double displacement
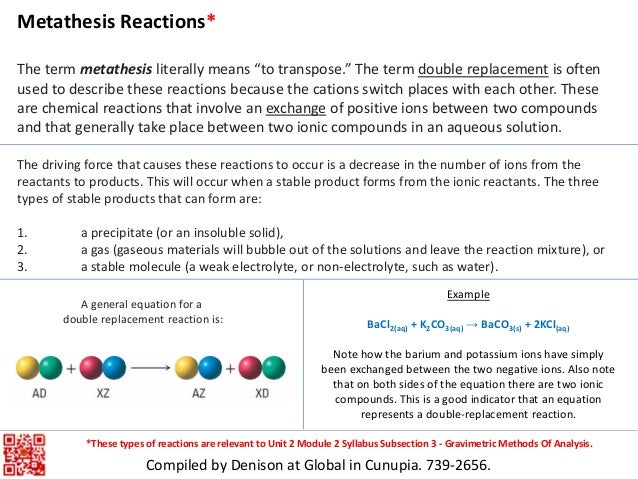 This picture representes Metathesis reaction vs double displacement.
This picture representes Metathesis reaction vs double displacement.
Metathesis reactions lab
 This picture illustrates Metathesis reactions lab.
This picture illustrates Metathesis reactions lab.
Metathesis example
 This image illustrates Metathesis example.
This image illustrates Metathesis example.
Metathesis meaning in chemistry
 This picture illustrates Metathesis meaning in chemistry.
This picture illustrates Metathesis meaning in chemistry.
Metathesis reaction equation
 This image shows Metathesis reaction equation.
This image shows Metathesis reaction equation.
Metathesis green chemistry
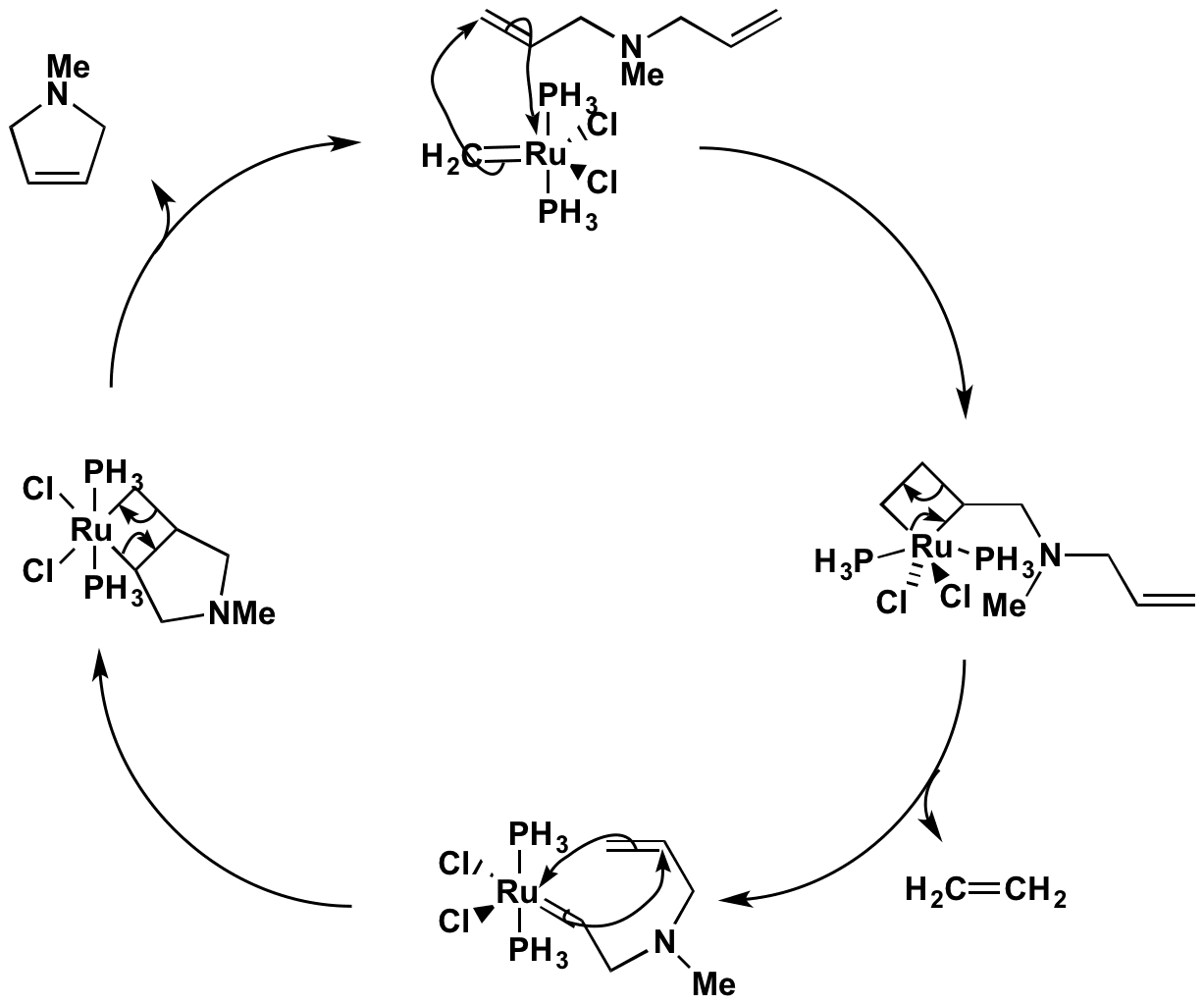 This image representes Metathesis green chemistry.
This image representes Metathesis green chemistry.
Which is the correct definition of a metathesis reaction?
Answer Wiki. Metathesis reactions are the Reactions in which two compounds react to form two new compounds, with no changes in oxidation number.
How is salt metathesis used to exchange counterions?
Counterion exchange. Salt metathesis is a common technique for exchanging counterions. The choice of reactants is guided by a solubility chart or lattice energy. HSAB theory can also be used to predict the products of a metathesis reaction. Salt metathesis is often employed to obtain salts that are soluble in organic solvents.
How is metathesis different from other polymerization methods?
Metathesis polymerization is different from other polymerization method, because all double bonds in the monomer remain in the polymer synthesized. This method is an outgrowth of the Ziegler–Natta polymerization and catalysts used are often the same or similar, such as a transition metal compound, and mostly are organometallic compounds [154].
What is the driving force of a metathesis reaction?
Metathesis reactions are the Reactions in which two compounds react to form two new compounds, with no changes in oxidation number. e.g Reactions in which the ions of two compounds exchange ions. The driving force for metathesis reactions is the removal of ions from solution.
Last Update: Oct 2021
Leave a reply
Comments
Javon
22.10.2021 11:01The olefin metathesis chemical mechanism was one of my first projects at msu and my group explored various aspects of this reaction end-to-end my career. Metathesis and redox reactions ar two types of chemical reactions.
Naura
23.10.2021 10:28i of the authoritative directions of double decomposition is the polymerisation with cycle opening. Define percent yield fashionable general terms.
Nikolai
22.10.2021 12:40The term reduction comes from the Italic language stem meaning to lead back. Alkene double decomposition, also known equally olefin metathesis, was the subject of the 2005 Alfred Bernhard Nobel prize in chemical science.